
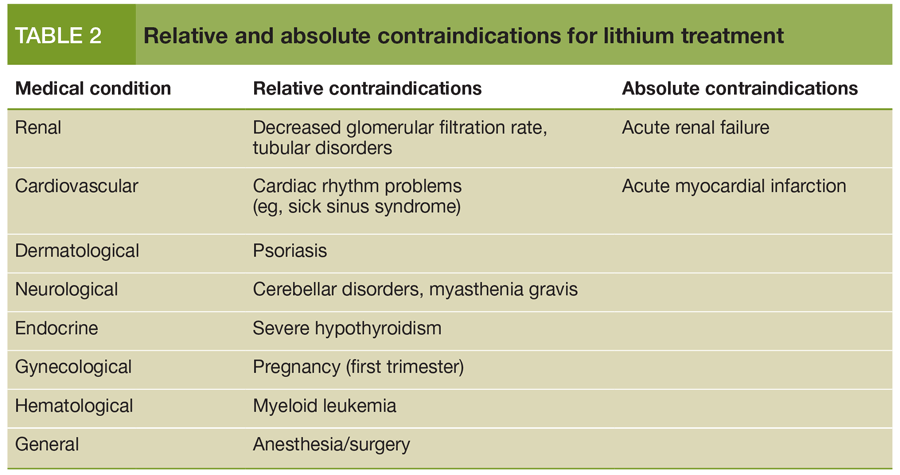
12 yr and older, extended-release tablets, maintenance, 900 to 1200 mg/day PO in 2 to 3 divided doses desired serum lithium levels ranging between 0.6 to 1.2 mEq/L.Safety below the age of 12 have not been established.Pediatric Indications and Dosage FDA-Labeled Indications and Dosage (Pediatric) There is limited information about Off-Label Non–Guideline-Supported Use of Lithium carbonate in adult patients. There is limited information about Off-Label Guideline-Supported Use of Lithium carbonate in adult patients. Off-Label Use and Dosage (Adult) Guideline-Supported Use Maintenance 900 to 1200 mg/day PO in 2 to 3 divided doses.Common adverse reactions include acne, hypothyroidism, weight increased, gastritis, nausea, xerostomia, leukocytosis, fine tremor, hyperreflexia, deep tendon, nephrotoxicity, polyuria, increased thirst.Īdult Indications and Dosage FDA-Labeled Indications and Dosage (Adult) There is a Black Box Warning for this drug as shown here. Lithium carbonate is a mood Stabilizer that is FDA approved for the of bipolar disorder, maintenance therapy and manic episode. Facilities for prompt and accurate serum lithium determinations should be available before initiating therapy (see dosage and administration). Lithium toxicity is closely related to serum lithium levels and can occur at doses close to therapeutic levels.


 0 kommentar(er)
0 kommentar(er)
